Illinois Tech Scientists Crack Code for Better Cold-Weather Batteries
Researchers at Illinois Institute of Technology have made an exciting breakthrough in battery technology by mapping the precise location of lithium atoms in a revolutionary new material. This discovery could lead to batteries that perform exceptionally well even in freezing temperatures, transforming everything from electric vehicles to energy storage systems.
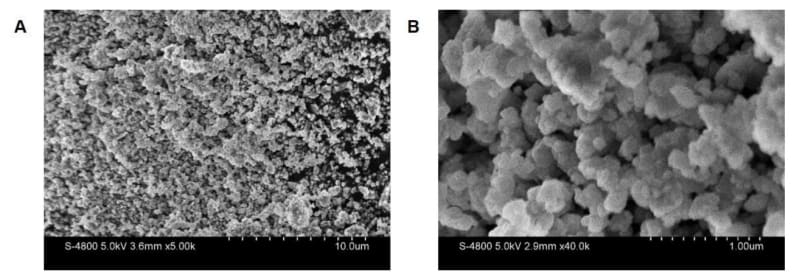
In a development that could change how we power our lives in cold climates, scientists at Illinois Institute of Technology have unlocked the secret to creating batteries that work beautifully even when temperatures drop. Their discovery centers on understanding exactly where lithium atoms live inside a promising new material called lithium tantalum oxychloride, or LTOC for short.
Research Professor James Kaduk played a crucial role in this breakthrough, though he describes his contribution with characteristic modesty as "small but useful." His passion, however, shines through when he talks about the work. "What really gets me excited is finding out where the atoms are," Kaduk says with infectious enthusiasm.
The challenge Kaduk faced was like trying to spot fireflies in a stadium full of searchlights. Lithium atoms are incredibly light, with only three electrons, making them nearly invisible to standard detection methods, especially when surrounded by heavier elements like tantalum. Rather than giving up, Kaduk got creative. He took an indirect approach, mapping out where the heavier atoms were located and then identifying the empty spaces between them where lithium could comfortably fit.
This clever detective work paid off beautifully. Kaduk discovered that the lithium atoms sit in special positions that are close enough together to allow them to "hop" easily from one spot to the next. Think of it like creating the perfect stepping stones across a stream, each one placed at just the right distance for an easy crossing.

The structure revealed something even more exciting: long, rigid chains of tantalum, oxygen, and chlorine that create open channels, like highways for lithium ions to travel through. These ions can zip along these pathways far more efficiently than in current battery designs, which means better performance all around.
The Ripple Effect
The implications of this discovery extend far beyond the laboratory. The ability of LTOC to conduct lithium ions effectively even in cold temperatures opens up transformative possibilities for communities in cold climates. Electric vehicles could maintain their range during harsh winters, a longstanding challenge that has limited adoption in northern regions. Energy storage systems could operate reliably year-round, supporting renewable energy infrastructure in places from Alaska to Scandinavia.
The research team confirmed their findings using sophisticated quantum mechanical calculations, and the structure held up beautifully. "The structure stayed very nearly the way it refined," Kaduk explains, "so that provided some extra evidence for the correctness of the structure."
For Kaduk, the satisfaction comes from solving the molecular puzzle using elegant, straightforward reasoning. When the quantum mechanics calculations confirmed his predictions about where the lithium atoms resided, it provided that extra boost of confidence that every scientist treasures.
This international collaboration, published in the prestigious journal Science, represents another step forward in humanity's quest for cleaner, more efficient energy storage. While Kaduk's piece may be one part of a larger puzzle, it's these careful, methodical contributions that ultimately unlock the innovations that improve our daily lives.
More Images



Based on reporting by Phys.org
This story was written by BrightWire based on verified news reports.
Spread the positivity! 🌟
Share this good news with someone who needs it
More Good News
DAILY MORALE
What did the thermometer say to the graduated cylinder?
EXPLORE INTEL
DAILY INSPIRATION
Hope is the thing with feathers that perches in the soul and sings the tune without the words and never stops at all.
Emily Dickinson


