Scientists Bring Real-World Chemistry Into the Lab with Brilliant Innovation
Researchers have developed an ingenious way to study materials at atmospheric pressure using standard laboratory equipment, eliminating the need for massive synchrotron facilities. This breakthrough could accelerate discoveries in clean energy, pollution control, and materials science by making advanced research accessible to more scientists worldwide.
In an exciting development that could democratize cutting-edge materials research, scientists have found a clever solution to a problem that has challenged researchers for decades. A team from ShanghaiTech University has successfully adapted standard laboratory equipment to study materials under real-world conditions, potentially opening doors for countless new discoveries.
Understanding how materials behave at the atomic level is crucial for developing everything from cleaner car engines to better batteries. Scientists have long relied on X-ray photoelectron spectroscopy (XPS) to peek at these microscopic surfaces, shooting X-rays at materials and analyzing the electrons that bounce back. This reveals precisely what elements are present and how they interact with each other.
However, there's been a significant hurdle. Traditional XPS requires a vacuum environment because air molecules interfere with the measurements. The challenge is that real-world chemical reactions happen at normal atmospheric pressure, creating what scientists call the "pressure gap." It's like trying to understand how a fish swims by observing it on land—you simply can't see its natural behavior.
Until now, bridging this gap meant traveling to enormous synchrotron facilities, which are stadium-sized and available to only a limited number of researchers. The waiting lists can be long, and access is competitive.

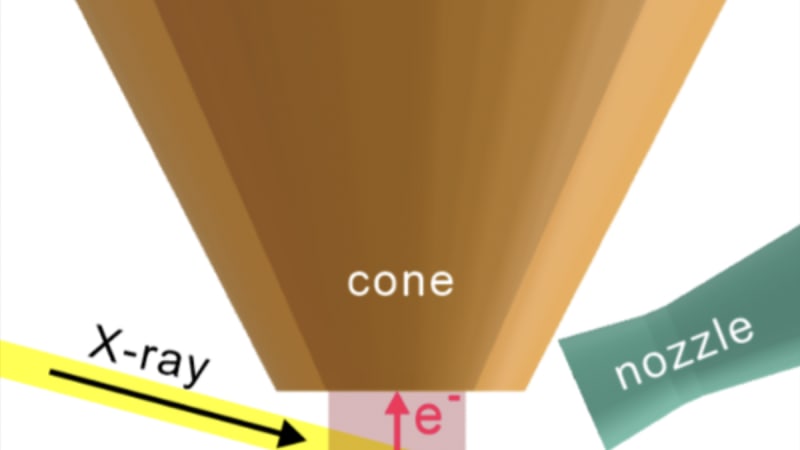
The breakthrough, published in Photon Science, brings this capability to standard laboratories. The researchers' ingenious solution involves technology borrowed from an unexpected source: rocket engines. They incorporated a de Laval nozzle, a specially shaped tube that pinches in the middle and flares at the ends, which accelerates gas to supersonic speeds.
By directing a focused jet of gas directly at the sample surface, the team created a tiny zone of atmospheric pressure right where it's needed. The fast-moving, concentrated gas maintains high pressure at the surface while the rest of the machine stays in vacuum, protecting the sensitive detectors. It's an elegant solution that works with existing equipment.
The researchers validated their approach through computer simulations and custom sensors, then tested it on platinum samples while spraying nitrogen gas. The results were remarkable: they successfully detected signals from both the gas and the metal surface at atmospheric pressure, something that would be impossible with conventional setups.
This innovation promises to transform materials research. Scientists will be able to study catalysts as they actually break down pollutants, watch rust form in real-time, and observe battery materials under working conditions—all in their own laboratories. No more waiting months for synchrotron access or traveling across continents.
The implications are inspiring. More researchers will have access to this powerful technology, potentially accelerating breakthroughs in clean energy, environmental protection, and industrial efficiency. Graduate students and scientists at smaller institutions will be able to conduct experiments that were previously out of reach.
As this technology proves itself and becomes more widely adopted, we can look forward to a future where understanding materials at the atomic level becomes routine rather than exceptional. It's a beautiful example of how creative engineering can remove barriers and expand the horizons of scientific discovery.
Based on reporting by The Hindu
This story was written by BrightWire based on verified news reports.
Spread the positivity! 🌟
Share this good news with someone who needs it
More Good News
 🚀 Innovation
🚀 InnovationAustralian Researchers Create Game-Changing Tool to Protect Water From Toxic Chemicals
 🚀 Innovation
🚀 InnovationJinkoSolar's AI-Powered Partnership Heralds Exciting Solar Energy Breakthrough
 🚀 Innovation
🚀 InnovationSK On and Seoul National University Make Exciting Battery Breakthrough
Joke of the Day
Why did the librarian get kicked out of class?
Explore Categories
Quote of the Day
"Do not go where the path may lead, go instead where there is no path and leave a trail."
— Ralph Waldo Emerson