Carnegie Mellon Finds New Path to Treat Muscular Dystrophy
Scientists at Carnegie Mellon University have discovered a breakthrough approach to target the root cause of myotonic dystrophy type 1, the most common form of adult muscular dystrophy. The precision therapy could finally offer hope to patients with a disease that currently has no effective treatment.
For the first time, researchers have found a way to stop the molecular traffic jam that causes the most common adult form of muscular dystrophy.
Scientists at Carnegie Mellon University developed tiny, precise molecules that can target the tangled RNA structures causing myotonic dystrophy type 1 without harming healthy cells. The breakthrough could help at least 1 in 2,300 people worldwide who live with this progressive muscle-weakening disease.
The condition happens when genetic code gets stuck on repeat. In healthy people, a specific sequence repeats 5 to 35 times, but in those with DM1, it can repeat thousands of times.
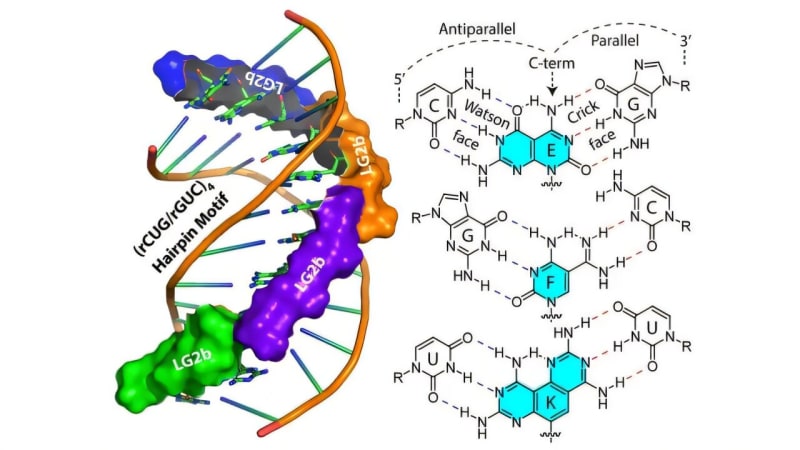
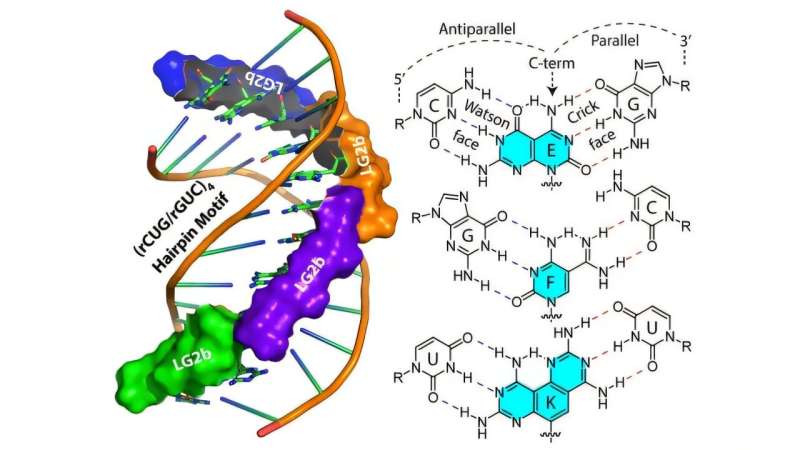
These repeats form hairpin loops that trap essential proteins needed for normal cell function. Without these proteins doing their jobs, cells can't produce the proteins needed for muscles to work properly.
"Diseases like myotonic dystrophy have complicated, life-stealing symptoms caused by the repeat of only three nucleobases, which seems so simple," said Danith Ly, a chemistry professor who led the research. "If we can stop proteins from being sequestered in this hairpin, we believe we can help improve the symptoms of these diseases."
Ly compares his team's solution to a pothole filler that repairs damaged spots without disturbing the rest of the road. The new molecules, called nucleic acid ligands, slip between the two strands of problematic RNA and hold them in place.

What makes this approach special is its precision. Traditional therapies struggle to tell the difference between healthy RNA and disease-causing RNA, often creating unwanted side effects.
The new ligands use a double-sided design inspired by Janus, the two-faced Roman god. This allows them to recognize and bind only to the specific harmful sequences while leaving normal RNA alone.
The Ripple Effect
This discovery extends far beyond one disease. The same approach could work for other conditions caused by RNA repeat expansions, including spinocerebellar ataxias, Friedreich's ataxia and ALS.
The research, published in the Proceedings of the National Academy of Sciences, opens doors that seemed locked just years ago. Ly's team at Carnegie Mellon's Center for Nucleic Acids Science and Technology has spent a decade developing this next generation of molecular tools.
In laboratory models, the lead molecule successfully targeted the disease-causing RNA. The team is now working to move this promising approach from the lab toward clinical trials.
For patients who currently face a diagnosis with no treatment options, this precision-targeting method represents real hope for disease-modifying therapies that could slow or stop progression.
The road from discovery to approved treatment takes time, but this breakthrough proves that even diseases caused by seemingly simple genetic stutters can be outsmarted with the right molecular strategy.
More Images

Based on reporting by Google News - New Treatment
This story was written by BrightWire based on verified news reports.
Spread the positivity! 🌟
Share this good news with someone who needs it