Scientists Use Egg White Chemistry to Double Battery Power
Researchers discovered that whipping protein foam like meringue creates tiny highways inside batteries, making them twice as powerful in the same space. This kitchen-inspired breakthrough could mean longer-lasting phones and electric cars that fit into sleeker designs.
The secret to better batteries might be hiding in your kitchen cabinet, and it could help solve one of the biggest problems holding back electric vehicles and smartphones.
Scientists just figured out how to make next-generation lithium-sulfur batteries pack twice as much power into the same space. Their solution came from an unexpected place: the fluffy meringue on top of pies.
Here's the challenge they faced. Lithium-sulfur batteries can store way more energy than today's standard batteries and cost less to make. But they're bulky, taking up nearly twice as much room as current batteries. In a world where every phone needs to be thinner and every electric car needs more range without getting bigger, that's a dealbreaker.
The problem happens inside the battery itself. Ions need to move through the battery material like cars driving through a city. Traditional designs create a maze of narrow streets and dead ends. To fix this, scientists usually make the batteries more porous, adding extra space for ions to move around. But all that extra space makes the batteries thicker and bulkier.
Researcher Petar Jovanović and his team asked a different question. Instead of making everything spacious, could they create dedicated highways for ions while keeping everything else tightly packed?
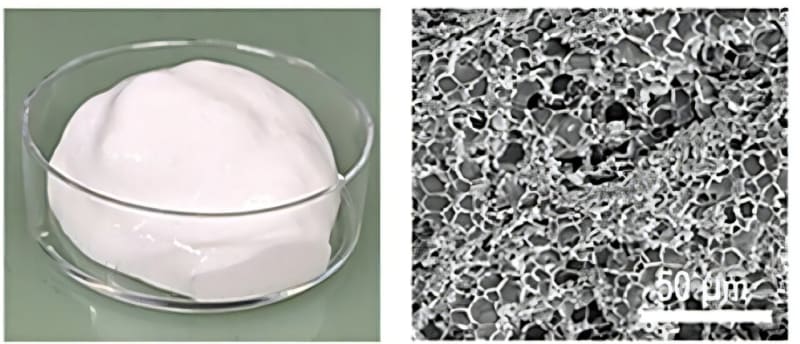
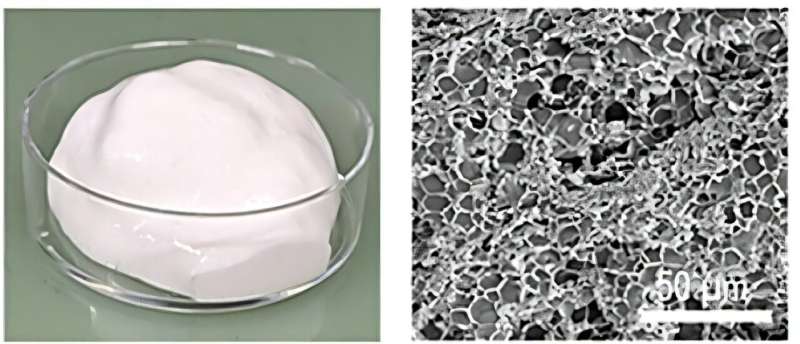
Their answer came from observing egg whites. When you whip egg whites into meringue, proteins trap tiny air bubbles in a delicate foam. The team wondered if those bubbles could become pathways for ions to travel.

They recreated this kitchen chemistry in the lab using cellulose and proteins similar to those in egg whites. When whipped together, these materials formed a tough, stretchy foam instead of a delicate dessert. They mixed this protein foam with sulfur and carbon, then coated it onto battery components.
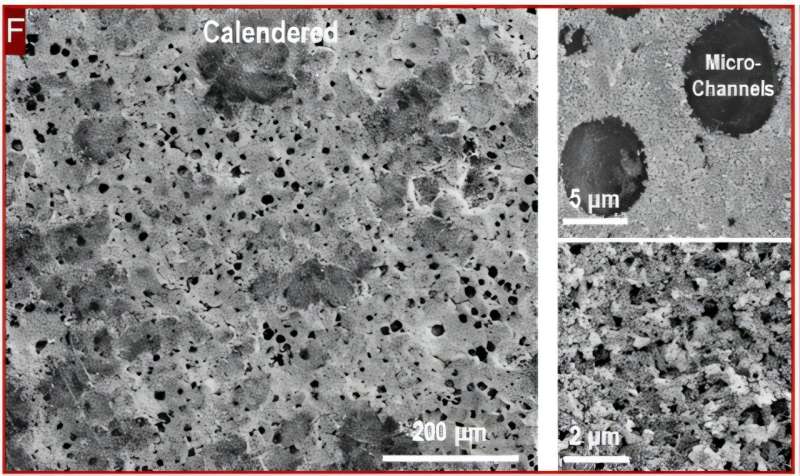
As the mixture dried, the bubbles collapsed and left behind narrow cylindrical channels. These channels stayed intact even when the team compressed the batteries to make them thinner, something that normally destroys battery performance.
The results surprised even the researchers. The batteries delivered double the volumetric performance they hoped for. Even better, they could charge in just 15 minutes while maintaining high capacity, something lithium-sulfur batteries typically struggle to achieve.
Why This Inspires
This breakthrough shows how solutions to complex problems can come from the simplest observations. A team of scientists looked at breakfast and saw the future of energy storage.
The research used cheap, readily available materials without complex manufacturing tricks. That means this technology could actually make it to market without requiring billion-dollar factories or rare ingredients.
For everyday people, this could translate into electric cars with longer range in smaller battery packs, phones that last all day in thinner designs, and lighter drones that fly longer. The technology brings us closer to a world where clean energy doesn't require compromise.
The work appeared in the journal Small Structures this month, moving from kitchen inspiration to scientific reality in a way that could reshape how we power our lives.
More Images
Based on reporting by Phys.org - Technology
This story was written by BrightWire based on verified news reports.
Spread the positivity! 🌟
Share this good news with someone who needs it


