Stanford's Silver Fix Makes Safer Batteries 5x Stronger
Stanford researchers cracked the code on solid-state batteries by coating them with silver thinner than a human hair. This simple treatment could finally unlock batteries that charge faster, last longer, and never catch fire.
A coating of silver atoms just three nanometers thick might be the breakthrough that brings super batteries to your phone and car.
Stanford University researchers discovered that treating ceramic battery cores with an ultra-thin layer of silver makes them five times more resistant to cracking. This solves one of the biggest problems holding back solid-state batteries, which promise to charge your electric vehicle in minutes instead of hours and store far more energy than today's lithium-ion batteries.
The challenge has stumped scientists for decades. Solid-state batteries replace the flammable liquid inside current batteries with a ceramic material that's safer and more powerful. But these ceramics crack like fine china, and those tiny flaws grow with each charge until the battery dies.
"A real-world solid-state battery is made of layers of stacked sheets," explained Wendy Gu, associate professor of mechanical engineering at Stanford. "Manufacturing these without even the tiniest imperfections would be nearly impossible and very expensive."
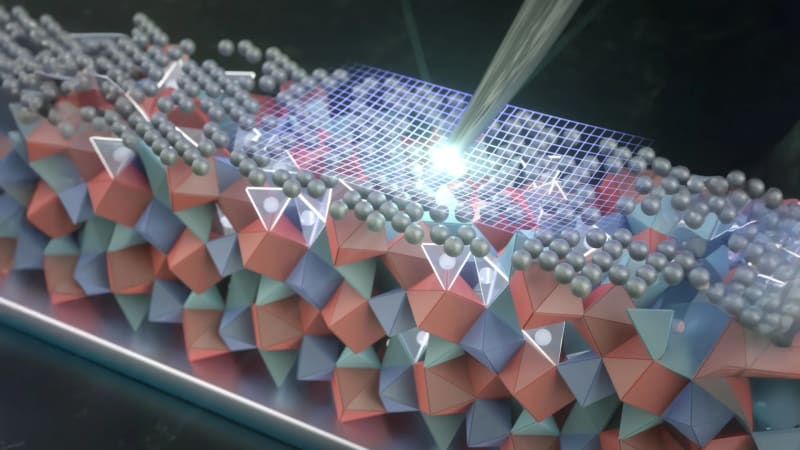
Instead of trying to make perfect ceramics, the Stanford team found a way to protect imperfect ones. They applied a silver solution to the surface and heated it to 300 degrees Celsius. The silver atoms migrated into the ceramic's crystal structure, replacing smaller lithium atoms and creating a protective shield extending 50 nanometers deep.
The silver stays in a positively charged form rather than becoming metallic. This turns out to be crucial for preventing cracks from forming and spreading. The coating also blocks lithium from pushing into existing flaws during fast charging, which normally causes the most damage.

Using a specialized probe inside an electron microscope, researchers measured exactly how much force the treated material could withstand. The silver-coated ceramic required nearly five times more pressure to crack compared to untreated samples.
"This method may be extended to a broad class of ceramics," said Xin Xu, who led the research and is now an assistant professor at Arizona State University. "It demonstrates ultrathin surface coatings can make the electrolyte less brittle and more stable under extreme conditions."
The team published their findings in Nature Materials on January 16. Their work builds on research from three years ago that revealed how microscopic cracks form and spread in these materials.
The Bright Side
The beauty of this solution lies in its simplicity. Instead of redesigning batteries from scratch or creating impossibly perfect materials, a whisper-thin layer of silver does the heavy lifting. The treatment works with existing solid-state battery designs and could apply to many types of ceramics beyond batteries.
The researchers are now testing the silver treatment on complete battery cells rather than small samples. They want to see if it maintains performance over thousands of charging cycles and works when scaled to full-size batteries.
If successful, this could be the final piece needed to bring solid-state batteries from the lab to your garage. Electric vehicles could charge as quickly as filling a gas tank. Phones might last days instead of hours. And the risk of battery fires could become a thing of the past.
Sometimes the biggest breakthroughs come from the smallest solutions.
Based on reporting by Science Daily
This story was written by BrightWire based on verified news reports.
Spread the positivity! 🌟
Share this good news with someone who needs it